R&D at Vetoquinol involves over 150 employees worldwide – from our Scientific Division, Research and Medical Division and Global Project Management Division. Every Vetoquinol project involves collaboration through the creation of multidisciplinary teams involving several departments. That’s because we know that working together is the best way to unlock new innovations.
As part of R&D at Vetoquinol, our researchers explore the paths which lead from the active ingredients to the animal. A large amount of creative work is necessary to make treatments as efficient and easy to administer as possible. Or, as we like to say, to make treatments ‘useful and usable’.
Pharmaceutical research, a long and complex process
R&D at Vetoquinol takes time. On average, the development of a new veterinary drug takes between 5 and 10 years and involves a multidisciplinary team, along with major investments of between €3-15million. As part of this process, galenic considerations are key. This means we only invent pharmaceutical formulas that are easy to use for vets, livestock farmers and pet owners.
By always looking for the best dosage, the best moment, the best place to administer the drug, Vetoquinol acts both for the safety of the animal and its owner. R&D at Vetoquinol also considers the profitability of livestock farmers – and how they can quickly put their animals back into production without any risk to consumers.
Some examples of our life-changing galenic formulations:
- Appetising pills, which are freely taken by cats and dogs.
- Single-dose injectable formulas with long-lasting effects, practical for injecting antibiotics in a herd.
- A dog treatment in liquid form, in a daily dose, easy for owners to administer accurately.

Marketing Authorisation, the ultimate objective of R&D at Vetoquinol
The marketing of any drug for humans or animals hinges on receiving marketing authorisation (MA). Granted on the basis of an exhaustive file, MA proves the product meets health authorities’ requirements in terms of quality, safety and efficacy. Once the product has been marketed, Vetoquinol continues to monitor the safety and efficacy of our drugs. We even have teams dedicated to drug monitoring and epidemiological surveillance.
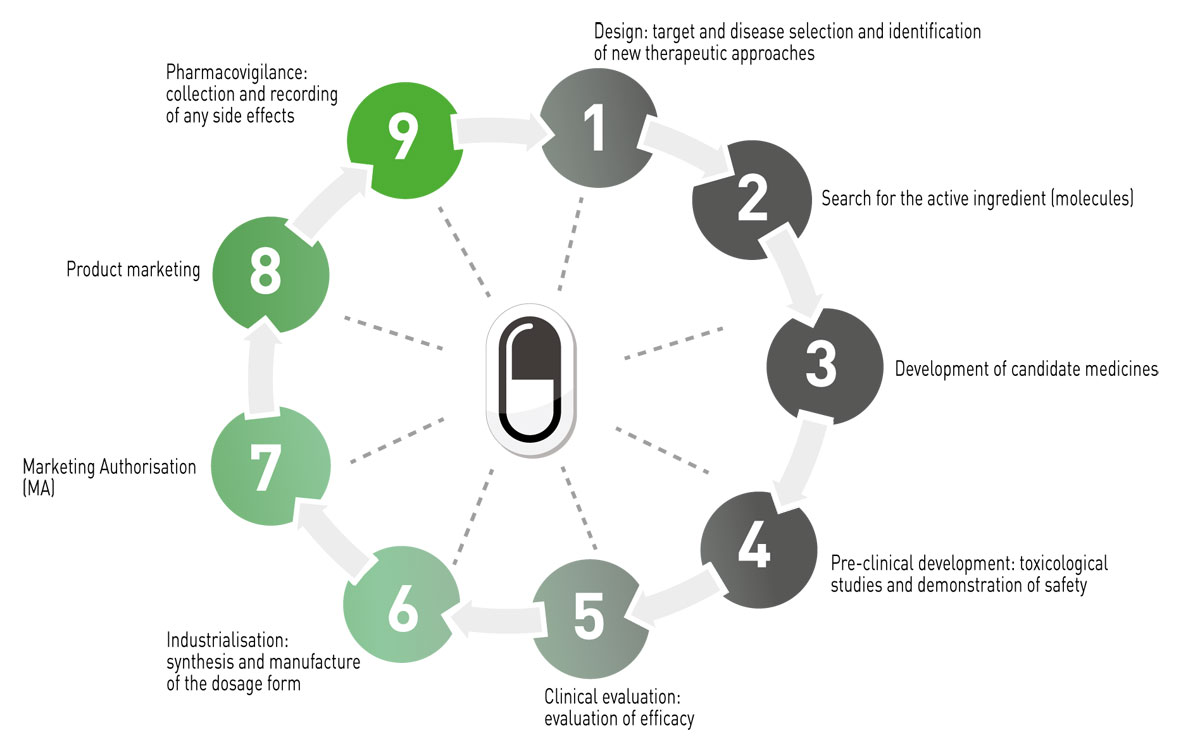
The main phases of veterinary drug development